Advancing Clinical Trials for Pharmaceutical Drug Development
Imaging-based insights
across trial pathways
AI-powered quantitative chest CT analysis is increasingly vital in respiratory clinical trials, providing non-invasive, precise, and objective assessment of anatomical abnormalities and disease progression to complement traditional endpoints.
Whether supporting primary, secondary, or exploratory endpoints metrics, LungQ® analyses enable in-depth exploration of treatment effects and mechanism of action.
With accurate, sensitive, and reproducible measurements, our advanced LungQ® ensures consistent, evidence-based insights across the entire trial pathway.
pre- clinical trial
Input for study phenotyping
and patient selection
phase 1
Early evidence on treatment
response patterns
phase 2-3
Quantitative data on treatment
efficacy and disease mechanisms
phase 4
Longitudinal evidence of sustained treatment outcomes
Why sponsors choose our analysis
Consistent output quality
handling large data sets variability across trial sites…
Strong clinical correlation
in comparison to conventional methods and clinical parameters
Extensive validation across diseases
in diverse patient populations from adult to pediatric age groups
Advanced QCT analysis with Artificial Intelligence
Combining high-fidelity segmentation and quantification capabilities, our LungQ® analysis measure lung structures, airway and vessel dimensions, and parenchymal density with regional detail. This enables precise, local assessment of emphysema, air trapping, bronchiectasis, mucus impaction, and related patterns, adding objective evidence alongside clinical and functional readouts.
Our areas of expertise include COPD, bronchiectasis disease, severe asthma, interstitial lung disease (ILD), cystic fibrosis, and other rare and common respiratory conditions, in both adult and paediatric populations.
Examples of QCT analysis with LungQ®:
Airway wall thickening
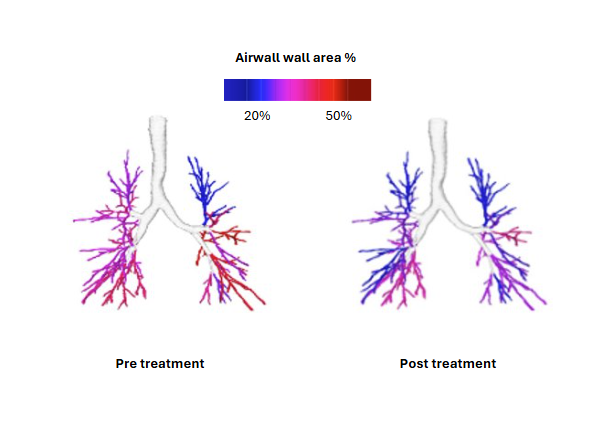
Airway wall thickening

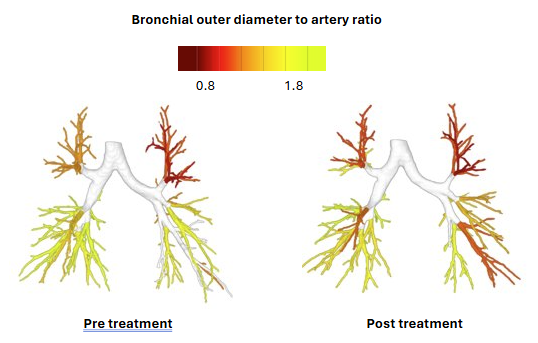
Airway narrowing/widening
Unlocking Full Imaging Potential
Beyond automated analysis, we offer enhanced expertise to help sponsors extract the full value from their imaging studies. From guiding protocol choices and standardizing acquisition to extending insights after AI analysis, our tailored support ensures optimized data quality, impactful results, and stronger trial outcomes.

Assessment of Treatment Efficacy of Cystic Fibrosis Therapies
Multiple validation studies evaluating the efficacy of elexacaftor/tezacaftor/ivacaftor (ETI), lumacaftor/ivacaftor (lum/iva)and inhaled hypertonic saline in paediatric CF patients, demonstrate high sensitivity and accuracy of LungQ’s measurements in quantification of disease changes over time.
Applied LungQ® metrics: bronchial wall thickening and widening, air trapping via changes in low-attenuation regions, mucus impaction and arterial and venous blood volume at baseline and follow up.

Assessment of Treatment Efficacy of Cystic Fibrosis Therapies
Multiple validation studies evaluating the efficacy of elexacaftor/tezacaftor/ivacaftor (ETI), lumacaftor/ivacaftor (lum/iva)and inhaled hypertonic saline in paediatric CF patients, demonstrate high sensitivity and accuracy of LungQ’s measurements in quantification of disease changes over time.
Applied LungQ® metrics: bronchial wall thickening and widening, air trapping via changes in low-attenuation regions, mucus impaction and arterial and venous blood volume at baseline and follow up.

Partnering for the shortest path to effective trials
In collaboration with established CROs and trial imaging management platforms, we can offer clinical trial sponsors the option to access Thirona’s LungQ® analyses through coordinated imaging workflows with their preferred providers, when specified by the protocol.
Sponsors can retain the operational efficiency of their existing data pipelines while requesting selected LungQ® measurements for advanced quantification of bronchial, parenchymal, and vascular structures. This integral service model supports fast, reliable delivery of reproducible quantitative outcome measures and helps minimize operational risk and time-to-market.
Partners we work with:














