Built on advanced AI capabilities in lung segmentation and quantification, our technology interprets thoracic CT data with anatomical precision - mapping airway trees, parenchymal patterns, and vascular structures, even in complex or severely diseased lungs. Anchored in the LungQ® platform, our proprietary technology supports the entire interventional pathway, from pre-procedural planning and intra-operative navigation to post-treatment assessment.
We partner with leading MedTech companies developing interventional devices, navigation technologies, and robotic bronchoscopy systems to advance the next generation of precision-driven pulmonary interventions.
Our AI-powered lung analysis methods enable accurate assessment and visualization of lung anatomy and pathology, supporting the development of image-guided interventional solutions.
Anatomical segmentation
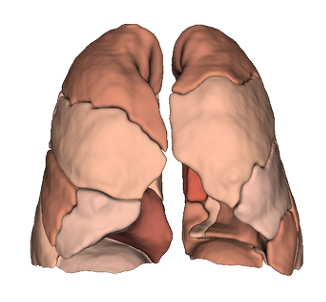
Lung lobes & segments
Anatomical segmentation

Pulmonary Segments
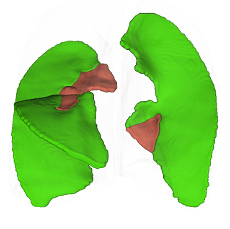
Anatomical segmentation
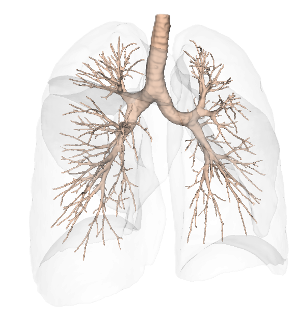
Bronchial Tree
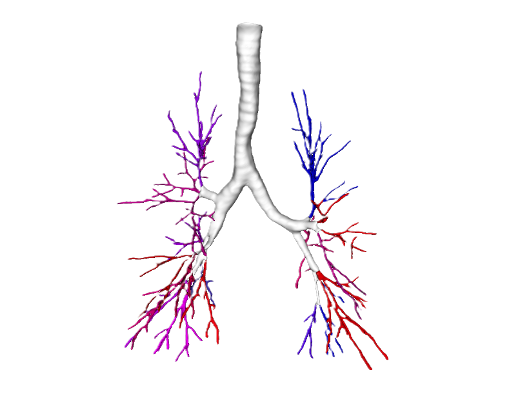
Anatomical segmentation
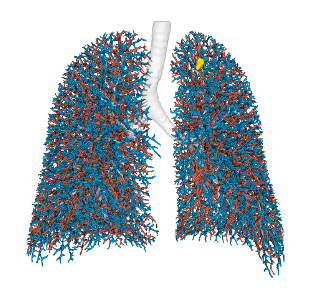
Pulmonary Arteries & Veins
Quantitative assessment
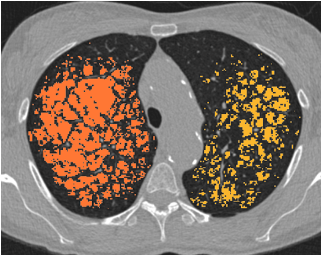
Emphysema severity
Quantitative assessment
Fissure completeness
Quantitative assessment
Air trapping/Hyperinflation
Quantitative assessment
Bronchial wall thickness
Quantitative assessment

Mucus plugging
Quantitative assessment

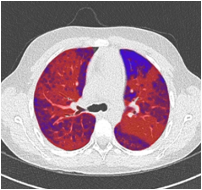
CT approximated perfusion defects
Quantitative assessment
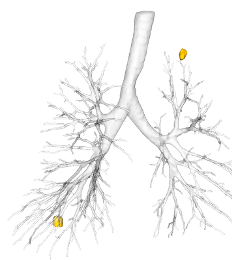
Lung nodule identification
Quantitative assessment

Bronchial narrowing/widening
Anatomical mapping
Bronchoscopic path planning
Anatomical mapping


Intra-operative image registration
These modular capabilities can be integrated into early R&D and further developed toward regulatory and clinical readiness.
LungQ® modules can be embedded into interventional systems and clinical workflows, enabling personalized procedural planning, real-time guidance, and longitudinal follow-up.
Powered by algorithms trained on disease-specific datasets, the modules deliver robust and consistent performance, even across variable scans and complex anatomies.
Building on these modular capabilities, we offer OEM-ready imaging solutions composed of combined high-performance capabilities from our advanced technology portfolio, purpose-built to support locally targeted bronchoscopic and surgical interventions across key therapeutic areas.
These solutions are designed for integration into clinical decision-support environments deliver quantitative, anatomically precise insights to support:
Developed and validated on disease-specific datasets, our solutions deliver reliable performance and lasting value, continuously refined through research and clinical collaboration.
Our software solutions are ready to be applied to a range of targeted treatment approaches, such as:
Emphysema / Hyperinflation
BLVR Implants | Parenchymal Ablation | Surgical Tissue Resection
Chronic Airway Disease
Mucus Clearance | Chronic Bronchitis Ablation
Lung Cancer
Peripheral Lung Nodule Biopsy | Tumor Ablation | Local Drug Delivery | Segmentectomy
*Clinical validation and regulatory clearance may vary per solution and region. Please contact us for detailed information regarding regulatory status and applicability to your use case.
Whether advancing next-generation therapies, enhancing image-guided navigation tools, or powering robotic bronchoscopy platforms, Thirona’s AI technology can be adapted to align with diverse development roadmaps and system architectures.
With flexible delivery models and a portfolio that includes both ready-to-integrate applications and custom-developable capabilities, we work with partners from early design stages through, clinical trials, regulatory and clinical implementation.
Chronic Bronchitis (CB) is characterized by excessive airway mucus production and is often associated with significant airway obstruction from mucus plugging. BR treatment is expected to address mucosal inflammation and hypersecretion by delivering pulsed electric fields to the airway mucosa.
By leveraging LungQ® MP analysis and Airway analysis, the treatment effect has been quantitatively proven evident on CT imaging: CB patients with CT-identified mucus plugging experienced a significant reduction in mucus plugs in 6 months following BR. This objective improvement directly aligns with patient-reported symptom relief, confirming the therapeutic mechanism.
Applied LungQ Metrics:

Multiple validation studies evaluating the efficacy of elexacaftor/tezacaftor/ivacaftor (ETI), lumacaftor/ivacaftor (lum/iva)and inhaled hypertonic saline in paediatric CF patients, demonstrate high sensitivity and accuracy of LungQ’s measurements in quantification of disease changes over time.
Applied LungQ® metrics: bronchial wall thickening and widening, air trapping via changes in low-attenuation regions, mucus impaction and arterial and venous blood volume at baseline and follow up.
